15 March 2012
15 March 2012
 Journal of Colloid and Interface Science. 370 - 1, pp. 73 - 79.
Journal of Colloid and Interface Science. 370 - 1, pp. 73 - 79.
 Sonia Losada-Barreiro*b, Verónica Sánchez-Pazb, Fátima Paiva-Martinsa, Carlos Bravo-Díazb, Laurence S. Romstedc
Sonia Losada-Barreiro*b, Verónica Sánchez-Pazb, Fátima Paiva-Martinsa, Carlos Bravo-Díazb, Laurence S. Romstedc
 Autor affilations:
Autor affilations:
*Corresponding authors
aREQUIMTE-LAQV, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade do Porto, 4169-007, Portugal
bDpt. Química Física, Facultad de Química, Universidad de Vigo, Vigo-Pontevedra, Spain
cDepartment of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, Piscataway, New Jersey 08854, United States
![]() E-mail: sonia@uvigo.es
E-mail: sonia@uvigo.es
Abstract
We determined the effects of emulsifier concentration and temperature on the distribution of gallic acid (GA) in a food-grade emulsion composed of 1:9 vol:vol stripped corn oil, acidic water and Tween 20. The distribution of GA can be defined by the partition constant between the aqueous and the interfacial regions, ![]() , which was determined by using a kinetic method and the pseudophase kinetic model. Once
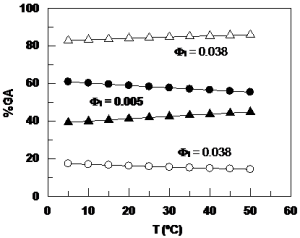
, which was determined by using a kinetic method and the pseudophase kinetic model. Once ![]() is known, determining the distribution of GA is straightforward. Our results show that at least 40% of the total GA is located in the interfacial region of the emulsion at 0.005 volume fraction of Tween 20, and this percentage increases to ca. 85% of the total GA at 0.04 volume fraction of Tween 20. The variation of
is known, determining the distribution of GA is straightforward. Our results show that at least 40% of the total GA is located in the interfacial region of the emulsion at 0.005 volume fraction of Tween 20, and this percentage increases to ca. 85% of the total GA at 0.04 volume fraction of Tween 20. The variation of ![]() with the temperature was used to estimate the thermodynamic parameters for the GA transfer from the aqueous to the interfacial region of the emulsion and the activation parameters for the reaction between 16-
with the temperature was used to estimate the thermodynamic parameters for the GA transfer from the aqueous to the interfacial region of the emulsion and the activation parameters for the reaction between 16-![]() and GA in the interfacial region. The free energy of transfer from the aqueous to the interfacial region,
and GA in the interfacial region. The free energy of transfer from the aqueous to the interfacial region, ![]() is negative, the enthalpy of transfer is small and negative, but the entropy of transfer is large and positive. Our results demonstrate that the partitioning of GA in acidic emulsions between aqueous and interfacial regions depends primarily on droplet concentration and is only slightly dependent on temperature.
is negative, the enthalpy of transfer is small and negative, but the entropy of transfer is large and positive. Our results demonstrate that the partitioning of GA in acidic emulsions between aqueous and interfacial regions depends primarily on droplet concentration and is only slightly dependent on temperature.