21 August 2007
21 August 2007
 Helvetica Chimica Acta. 90, pp. 1559 - 1573.
Helvetica Chimica Acta. 90, pp. 1559 - 1573.
 Sonia Losada-Barreiroa, Verónica Sánchez-Paza, Carlos Bravo-Díaz*a
Sonia Losada-Barreiroa, Verónica Sánchez-Paza, Carlos Bravo-Díaz*a
 Autor affilations:
Autor affilations:
*Corresponding authors
aDpt. Química Física, Facultad de Química, Universidad de Vigo, Vigo-Pontevedra, Spain
![]() E-mail: cbravo@uvigo.es
E-mail: cbravo@uvigo.es
Abstract
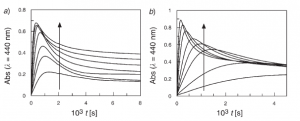
We have investigated the kinetics and mechanism of the reaction between 3-methylbenzenediazonium ions (3MBD) and methyl gallate (=methyl 3,4,5-trihydroxybenzoate; MG), in aqueous buffer solution by employing spectrophotometric (UV/VIS) and electrochemical (linear-sweep voltammetry, LSV) techniques and computational methods. Because the absorption band of MG overlaps that of 3MBD, the reaction was monitored spectrophotometrically by measuring the changes in absorbance with time due to product formation, and biphasic kinetic profiles, indicative of accumulation of an intermediate in the course of the reaction, were obtained. The formation of an intermediate was confirmed by LSV. The observed rate constants kobs for 3MBD disappearance were obtained by fitting the decrease in the peak current of the first reduction peak of 3MBD with time to the integrated first-order equation. The variation of kobs with [MG] was determined at different pH values and follows a saturation kinetic pattern. Alternatively, at a fixed [MG], kobs values show an inverse dependence on [H+], suggesting that the reactive species is the anion and not the neutral form of MG. To discern which of the three OH groups of MG is the first one undergoing deprotonation, the geometries of the resulting anions were optimized by using B3LYP hybrid density functional theory (DFT) and a 6-31G(++d,p) basis set. The deprotonation energies suggest that the OH group at the 4-position is first deprotonated. The kinetic results can be accommodated, therefore, by assuming two competitive mechanisms, the spontaneous DN+AN decomposition involving 3MBD, and a mechanism involving an electrophilic attack at the O-atom at C(4) in a pre-equilibrium step, leading to the formation of a transient diazo ether of the type Ar![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) N
N![[DOUBLE BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8fe.gif) N
N![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) O
O![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) R which further decomposes. All attempts to isolate and characterize the diazo ether failed.
R which further decomposes. All attempts to isolate and characterize the diazo ether failed.