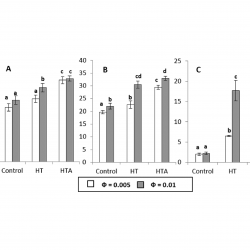
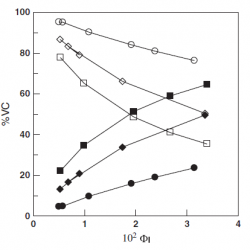
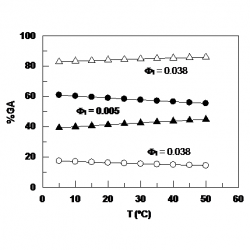
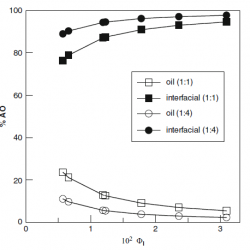
Distribution of Hydroxytyrosol and Hydroxytyrosol Acetate in Olive Oil Emulsions and Their Antioxidant Efficiency
We employed a kinetic method to determine the distributions of the antioxidants hydroxytyrosol (HT) and hydroxytyrosol acetate (HTA) between the oil, aqueous, and interfacial regions of a model food emulsion composed of stripped olive oil, acidic water, and a blend of Tween 80 and Span 80 [hydrophilic–lipophilic balance (HLB) = 8.05] as an emulsifier [...]