Encapsulation and solubilization of antioxidants gallic acid and ethyl, propyl and butyl gallate with B-cyclodextrin
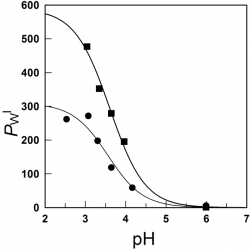
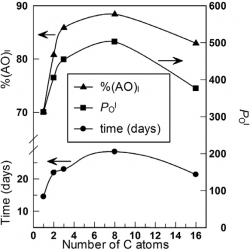
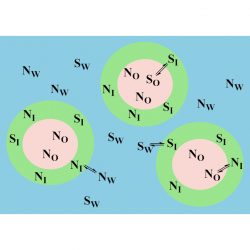
We investigated the formation of inclusion complexes of the antioxidants (AOs) gallic acid, GA, and its hydrophobic derivatives ethyl (EG), propyl (PG) and butyl (BG) gallate with β-cyclodextrin (β-CD) by employing the UV spectral shifts method and, for the sake of comparisons, a modified phase-solubility method that exploits the use of a competitive guest molecule (acetonitrile, ACN) that completely removes the antioxidants from the CD cavity and, at the same time, keeps them in solution [...]