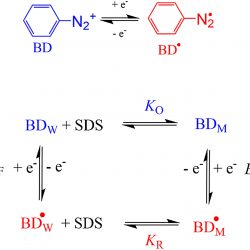
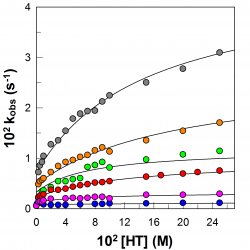
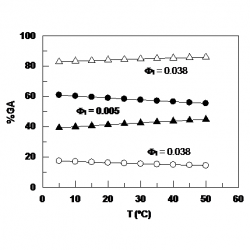
Partitioning of aryl radicals in micellar systems
Determining the association constants of radicals to biomimetic systems is not a simple task because of the inherent experimental difficulties associated to their “in‐situ” generation together with their high chemical instability, requiring the use of radical trapping agents in combination with, for example, magnetic (eg, EPR) and/or time‐resolved techniques (eg, frequency comb spectroscopy) to indentify and quantify them. Here, we have exploited the unique electrochemical properties of arenediazonium ions, ArN2+, to estimate the association constant of the electrochemically generated aryl radicals derived from benzenediazonium ions, BD, with sodium dodecyl sulfate (SDS) micelles [...]